A. Goals and Objectives: Copper(I/II) and zinc(II) are essential metal ions for living organisms involved in a variety of vital processes ranging from respiration, oxidative protection, tissue growth, metabolism of cholesterol and glucose, to the reading of the genome. To provide these functions, these metal ions are selectively incorporated as cofactors into the active sites of enzymatic proteins typically via the thiolate groups of Cys and the imidazole group of His, or more rarely the thioether group of methionine, the carboxylate groups of Glu and Asp, or the N or O of backbone amide groups. Because of the similarity of these coordination groups, an intriguing question is how do the His and Cys ligands selectively incorporate either Cu(I/II) or Zn(II) to function correctly?
B. Research goals are: (1) to study copper(I/II) and zinc(II) binding of a series of alternative metal binding (amb) peptides, which have potential as therapeutics for alleviating disease where abnormal metabolism of copper concentrations are involved, such as in Wilson’s and Menkes disease; (2) to study the metal binding characteristics of the methanobactin peptide produce by methanotrophic bacteria; (3) to train students in cutting-edge molecular techniques for elucidating the interrelationship between copper(I/II) and zinc(II) binding selectivity and structural functionality of oligopeptides; and (4) to integrate these research activities with the education and training of undergraduate and Master’s students.
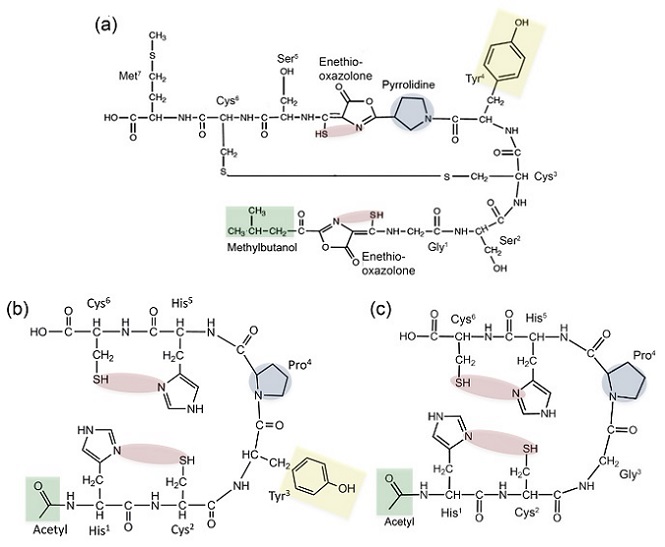
Figure 1. The comparison of the structures of methanobactin produced by Methylosinus trichosporium OB3b (mb-OB3b) and the alternative metal binding peptides amb1 and amb2. Presented structures represent the neutral charged state of each molecule. (a) Structure of mb-OB3b with the sequence 1-(N-[enethiol-(5-oxo-2-(3-methylbutanoyl)oxazol-(Z)-4-ylidene)methyl]-Gly1–Ser2–Cys3–Tyr4)-pyrrolidin-2-yl-(enethiol-[5-oxo-oxazol-(Z)-4-ylidene]methyl)–Ser5–Cys6–Met7 showing the 2N2S enethiol-oxazolone binding sites (shaded in red), the pyrrolidine allowing the tight turn (shaded in blue), the N-terminal methylbutanol (shaded in green), and the tyrosine residue (shaded in yellow). (strong) Structure of acetyl–His1–Cys2–Gly3–Pro4–His5–Cys6, (amb1) showing the 2N2S of the His and Cys substituent groups (shaded in red) substituted for enethiol-oxazolones, the proline (shaded in blue) substituted for the pyrrolidine, the acetyl group (shaded in green)blocking the N-terminal mimicking the methylbutanol group of mb-OB3b. (c) Structure of acetyl–His1–Cys2–Tyr3–Pro4–His5–Cys6, (amb2) the His and Cys substituent groups (shaded in red), the proline (shaded in blue), the acetyl group (shaded in green), and the tyrosine residue (shaded in yellow). The amb peptides are polyprotic systems and include a carboxyl group at the C-terminal, two His imidazole groups, two Cys thiol groups, and Tyr phenol group with pKas of 2.0, 6.0, 8.4 and 11.0, respectively, based on the pKas of the amino acids. These polyprotic sites result in ambs predominantly forming positive charged ions at pH < 6.0 and negative charged ions at pH > 6.0.
C. Research Activities: Methanobactins (mbs) are a class of small molecules with high copper(I) binding affinity. They are secreted by methane-oxidizing bacteria, known as methanotrophs, and their structural variations are known to affect their metal-binding affinities. Our research uses our expertise in instrumental analyses to achieve 3 objectives: (1) design and synthesize copper-binding peptides based on 2His-2Cys coordination sites and the mb-OB3b peptide from Methylosinus trichosporium OB3b; (2) conduct a series of gas-phase, solution-phase, and in silico-phase studies of ambs and mb-OB3b to elucidate the interrelationship between metal ion selectivity, redox activity, oligomerization and structural functionality; and (3) develop new ion mobility - mass spectrometry (IM-MS) methods for studying the solution-phase reactions of molecules of biological importance.
The team focuses on:
D. Research Development: The research develops IM-MS-based techniques for studying solution-phase peptide redox activity and selectivity toward copper(I/II) or zinc(II). The research contributes to fundamental knowledge on which sequence properties, substituent groups, and binding sites are favorable for copper(I/II) or zinc(II) binding and the accompanying redox and oligomerization reactions. The research also advances current knowledge of IM-MS and fluorescence spectroscopic techniques for analyzing solution-phase chemistry.
To request a change to this page or to request access to make changes yourself, email helpdesk@tamuc.edu.

